Data Integrity & Analytics for Biotech & Pharma Manufacturers
Struggling with scattered process data and strict compliance requirements? Lucid Automation & Security helps California biotech and pharma manufacturers turn GMP data into a compliant, valuable asset. We design and implement robust data management systems that ensure your information is complete, accurate, and ready for audits, while empowering your team with real-time insights.
As a company based in the San Francisco Bay Area, we specialize in the life sciences sector. Offering the rare blend of automation expertise, integration know-how, and GMP compliance experience needed to keep your operations running smoothly.
We treat your data with the same importance as the products you manufacture, maintaining its integrity from collection to archive. Our Data Integrity & Analytics services cover everything from historian integration and alarm management to electronic batch records and AI/ML-ready data pipelines.
By bridging the gap between the plant floor and enterprise systems, we eliminate data silos and manual workarounds. This results in accessible, trustworthy data that drives better decisions, ensures regulatory compliance, and ultimately accelerates your innovation.
Key Benefits of Our Data Solutions
Audit-Ready Compliance
We design systems with 21 CFR Part 11 and ALCOA+ principles in mind, ensuring data is attributable, secure, and easily retrievable for GMP inspections. No more scrambling during audits – your electronic records and audit trails are always in order.
Single Source of Truth
By integrating process historians and databases, we centralize all your critical production data. This means an end to disparate spreadsheets and databases. You get one reliable source for trends, reports, and analytics.
Improved Batch Release
Implement electronic batch records (eBRs) and automated reporting to speed up batch reviews. With features like review-by-exception, your quality team can release batches faster when all criteria are met, avoiding tedious manual checks.
AI/ML-Ready Data:
We help condition and contextualize your datasets for artificial intelligence and machine learning initiatives. By cleaning, structuring, and contextualizing data, we ensure that advanced analytics tools can actually generate meaningful, validated insights from your process data.
Historian Integration & Alarm Management
Process Historian Services: Historical data collection and analysis are the backbone of continuous improvement in regulated industries. We deploy and configure robust process historians (such as OSIsoft PI, Rockwell FactoryTalk Historian SE, and AVEVA/Wonderware InSQL) to automatically log your critical process parameters.
Our team ensures these historians are properly integrated with your control systems (PLC/SCADA/BMS) so that every data point is captured in real time with timestamps and context.
By having a centralized, scalable database of time-series data, you can easily trend batches, analyze deviations, and generate reports that stand up to scrutiny.
Alarm & Event Tracking: Tired of alarm fatigue or losing track of who acknowledged what alarm when? We implement alarm and event databases that record every alert and operator action. This service provides a clear timeline of events and alarm histories across your facility.
With proper alarm management tools, your team can perform rationalization (to cut down nuisance alarms) and analyze patterns to improve safety and uptime. Crucially, a complete alarm log is also a compliance asset. It shows inspectors that critical events are documented and addressed promptly.
Lucid configures alarm management systems to integrate with your historians and HMIs, so alarms are contextualized alongside process data for a complete picture. The result is faster troubleshooting (since you can correlate alarms with process conditions) and a database that supports continuous improvement of alarm settings.
Electronic Batch Records (EBR) & Reporting
Paper-based batch records and manual data entries can slow down production and invite errors. Our Electronic Batch Records (EBR) solutions replace paper binders with secure, digital records that capture every step of your production process.
We can leverage your existing historian and SQL databases, to configure batch record workflows that are user-friendly for operators and 100% traceable for compliance. Each addition, parameter change, and process checkpoint is automatically logged with user credentials and timestamps, building an audit trail that meets FDA GMP requirements. By implementing EBR systems, we help you achieve “review by exception.” That means if a batch runs flawlessly within spec, the system can highlight a one-line release approval (with all supporting data available on demand), rather than making quality reviewers slog through stacks of data.
Your team spends less time flipping pages and more time on value-added activities. And if there’s a deviation, it’s immediately flagged with all relevant data, so investigations are quicker and more thorough.
Example of a batch report dashboard summarizing key production data for an electronic batch record. Critical values, operator notes, and an alarm summary are displayed in context, enabling release-by-exception when all criteria are met.
Lucid’s Data Integrity & Analytics team designs custom reports like this to distill complex batch data into an easily reviewable format. By presenting only the essential information (and making full details available as needed), we help streamline batch release and improve confidence in the process data.
In addition to EBR implementation, Lucid can integrate reporting tools such as SQL Server Reporting Services (SSRS), Rockwell Optix, Dream Report, or Power BI to deliver the insights you need.
Whether you require automated batch summaries, monthly quality trend reports, or ad-hoc data analysis, we ensure that generating those reports is as simple as clicking a button. The reports will be accurate, timely, and tailored to your operations. No more exporting data to Excel and manually piecing together charts.
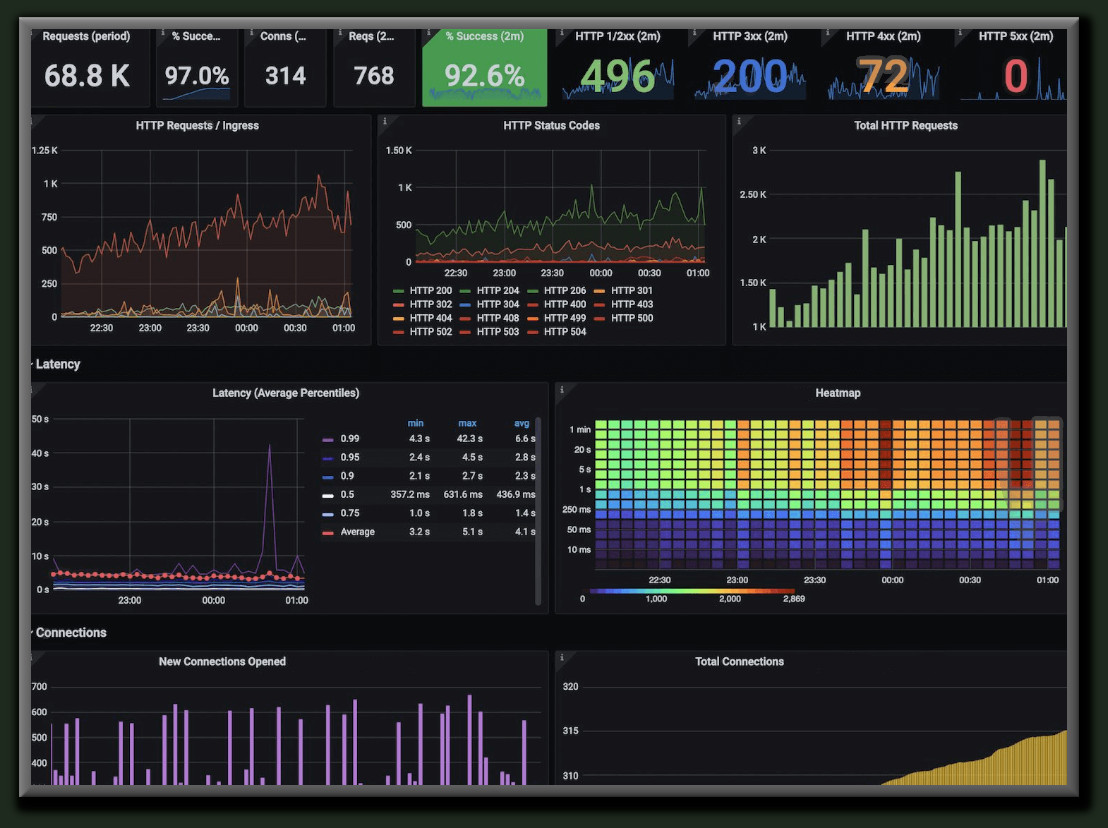
Data Visualization
Dashboarding & Contextual Data Display
Data is only useful if the right people can easily interpret it. That’s why we build custom dashboards and intuitive visualizations to bring your production and business data to life. Lucid Automation & Security can develop role-based dashboards for operators, engineers, managers, or executives – so each user sees the information that matters most to them, in a format that’s clear and actionable.
For example, a process engineer might have a dashboard showing real-time trends of critical process parameters with overlays of control limits, so they can spot anomalies at a glance. We incorporate contextual data display, meaning we add context like units, setpoints, batch phase names, or alarm descriptions so that data is immediately meaningful.
Lucid designs graphical, easy-to-navigate interfaces that aggregate data from historians, databases, and live equipment.
Our team is experienced with a variety of visualization and business intelligence tools, from traditional HMI/SCADA screens to web-based dashboards and platforms like Power BI or Ignition Perspective. We also ensure that these dashboards are securely accessible – whether on the plant floor or remotely – with proper user access controls.
Plant-Floor to Enterprise Data Integration
Manufacturing data doesn’t belong in a silo. We help connect your plant-floor systems to enterprise-level systems, ensuring a seamless flow of information across your organization. Lucid Automation & Security acts as the translator between Operational Technology (OT) and Information Technology (IT) – we make sure your machines and controllers can communicate with your business applications in a secure and structured way.
Our integration services include configuring OPC UA servers, MQTT brokers, and other middleware that let different systems share data reliably. For instance, we can set up data handshakes between your process historian/SCADA and your MES (Manufacturing Execution System) or LIMS (Laboratory Information Management System). This allows production data to automatically feed into electronic batch records or quality systems. We also integrate with higher-level systems like ERP or cloud analytics platforms – pushing key metrics (yields, downtime, throughputs) to where finance or supply chain teams can use them, and pulling down setpoints or schedules from planning systems to the plant floor when needed.
The benefit of this IT/OT integration is enterprise-wide visibility and streamlined operations. Imagine your maintenance software automatically creating a work order when a critical sensor shows a failure prediction, or your inventory system updating in real time as batches are completed. We design integration solutions tailored to your needs, using industry standards and best practices for security (data encryption, network segmentation, user authentication) so that data moves efficiently but safely. With Lucid’s help, you can break down the barrier between production and enterprise systems, allowing your company to be truly data-driven at every level.

