Your Shortcut to
Audit-Ready Compliance
Get a complete suite of editable templates for every step of your computer system validation lifecycle:
URS – User Requirements Specifications
FRS – Functional Requirements Specifications
SDS – System Design Specifications
Trace Matrix – Requirements-to-test mapping
FAT & SAT – Factory and Site Acceptance Test protocols
IQ/OQ – Installation & Operational Qualification
Why You’ll Love It
GAMP 5 Aligned: Built to fit industry best practices and regulator expectations
Time Savings: Slash authoring time by up to 70 % with pre‐written content
Consistency: Standardized format and language speed peer reviews
Audit-Ready: Includes version control logs and approval workflows
Fully Customizable: Word and Visio files let you adapt to your process
Stop wrestling with documents and get back to science.
Simplify Your GMP Validation with Code Modules
Simplifying Acceptance Testing starts with design docs and software developed with a solid foundation of GMP change control. There are many ways to write process control code, but leveraging certain tools like code modules and parameter UDTs can greatly reduce both the initial validation effort and future system modifications.
Ask us how we can help manage the validation effort for your next automation control project.One biopharma project I was on saved over 300 pages of testing by replacing the traditional PLC & HMI structure with a set of AOI’s in about 8 lines of a structured text ‘FOR’ loop. This let us heavily leverage parameter files for indirect HMI display addressing. Combining these techniques, we had fewer custom links to validate leading to a reduced Data Display, Data Entry, Alarming, and Security testing required.
While I cherry-picked this example from my experience in a highly regulated industry, it highlights the possible effectiveness and savings that may be achieved by selecting the right tool. All projects are unique and this is just one of many efficiency techniques in your toolbox.
However, if engage the Validation/Quality groups early in a project, you have more opportunity to find a clever solution early and make everyone’s job easier.
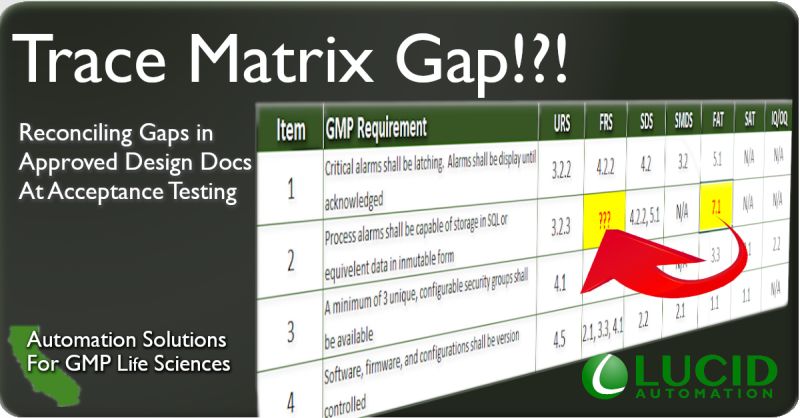
Trace Matrix Gap
Inevitably, we all run into a documentation gap. Restarting the workflows to initiate the change, approve, test, and review can add weeks to go back to design documents. Alternatively, it may be possible to just accept the gap and note it will be resolved in a follow up project.
It really depends on the size of the project, complexity, and sophistication of your vendors.
Contact us to help navigate through tough GMP validation situations such as these.
Future Proof Your Compliance System
Validating Biotech AI
One of our more aspirational career goals has been efficient validation testing. It’s an odd goal, but one those that have signed a thousand test forms can relate. Automation testing is an absolute necessity in a biopharma setting to increase quality for patients and hold stakeholders/vendors accountable. In practice, however, many validation efforts still fall into a quagmire of paperwork exercises and overlook their original intent.
Put on your seat belts, this is going to get technical fast.
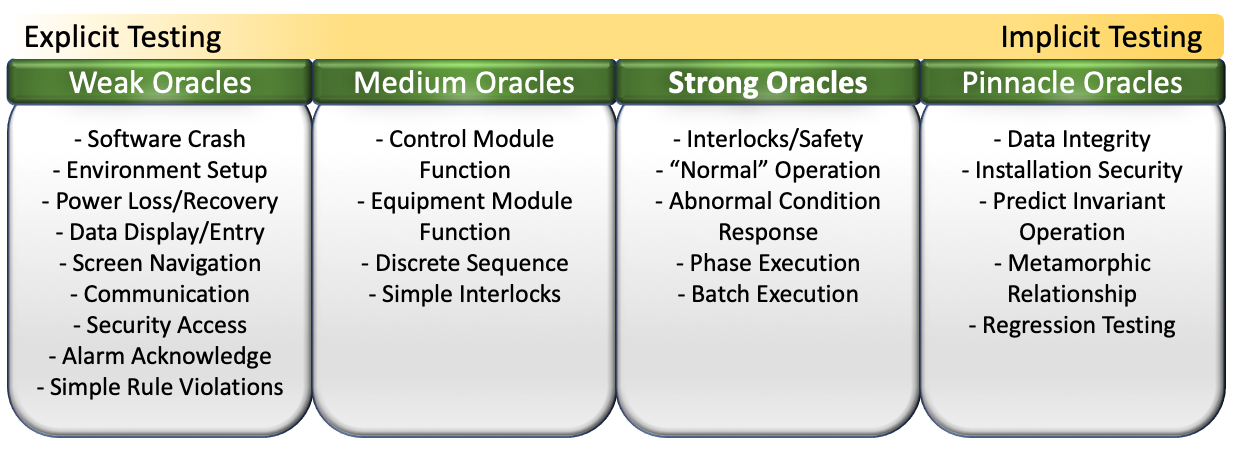
The current generation of tools we’ve developed are based on automated software testing techniques established in the early 80’s using test ‘oracles’. An oracle is just the authority used to determine if a test passes or fails. The oracle knows the right output for an expected input. [more…]